Background
Acute myeloid leukemia (AML) is an aggressive hematologic malignancy characterized by rapid disease progression and poor clinical outcomes. Mutations in FLT3 (Fms-like tyrosine kinase 3) are among the most common genetic abnormalities in AML, contributing to increased relapse rates and reduced survival. As a result, FLT3 represents a critical therapeutic target for AML treatment. However, the conventional discovery and optimization of FLT3 inhibitors are resource-intensive and time-consuming, requiring extensive efforts to achieve the necessary balance between potency and selectivity.
The Inventum.AI platform accelerates the drug discovery process by integrating advanced machine learning techniques with best practices in molecular modeling. This case study describes the design and validation of a novel FLT3 inhibitor using the platform’s Scaffold Generation and Periphery Generation modules, enabling rapid identification and optimization of promising candidate molecules.
Objective
The primary objective of this study was to design and validate a potent and selective FLT3 inhibitor for the treatment of AML. Utilizing a hybrid AI and structure-based drug design (SBDD) approach, we aimed to generate novel molecular structures with favorable potency, selectivity, and synthetic feasibility.
Methodology
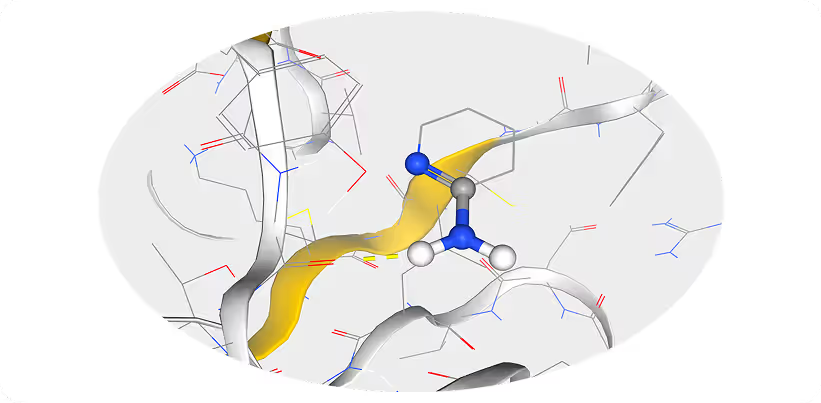
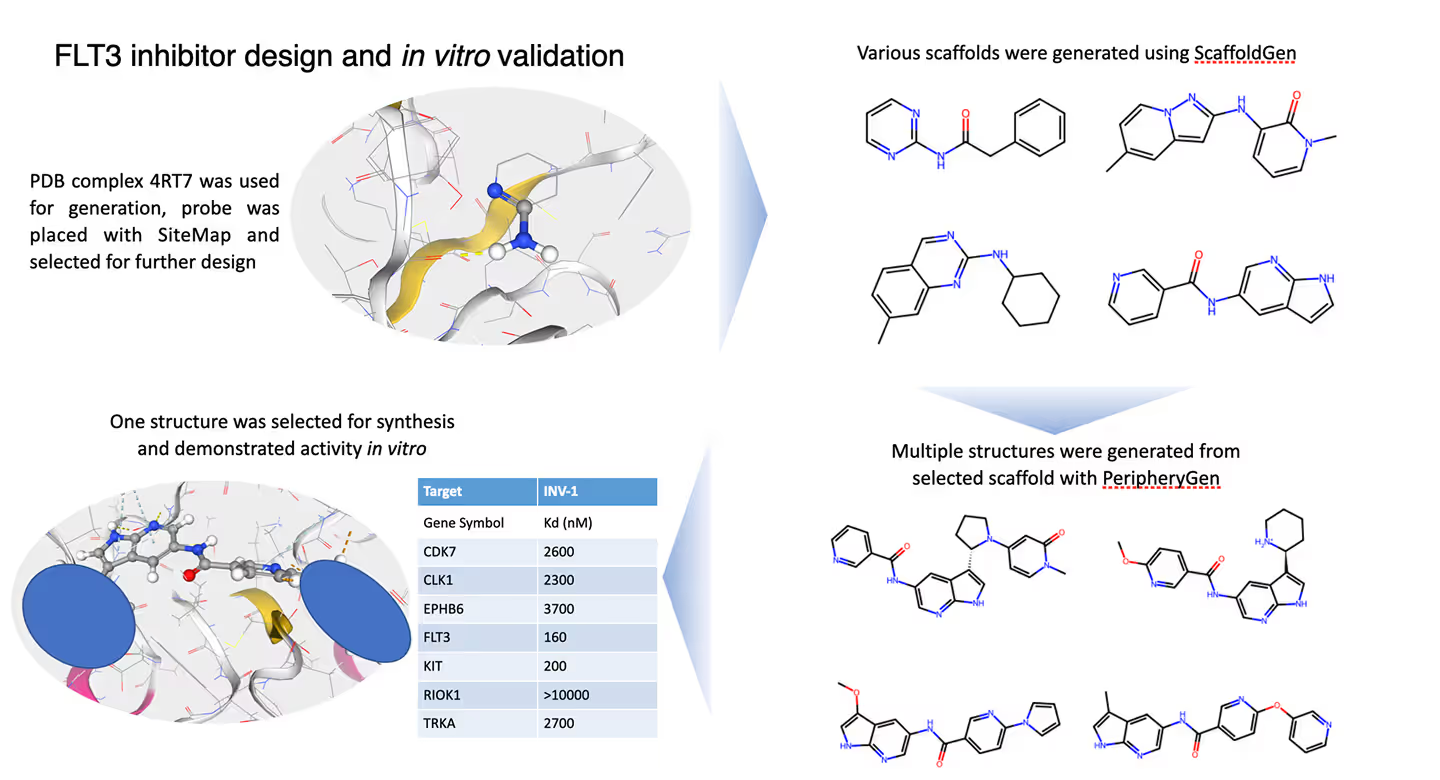
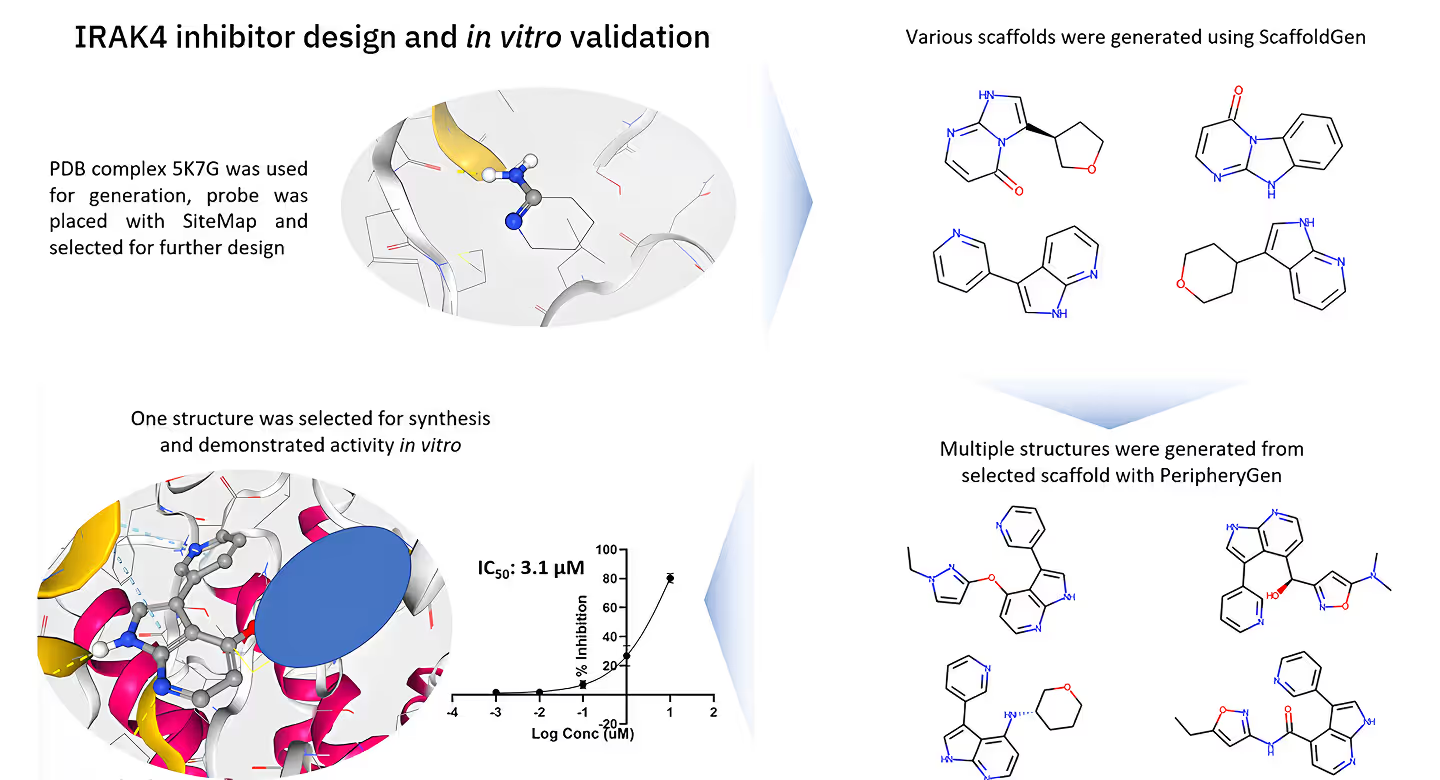
Model Preparation:- • We selected the known FLT3-ligand complex (PDB ID: 4RT7) as the structural basis for our study.
- • Using the SiteMap tool, we positioned an aminopyridine fragment within the FLT3 binding site to identify key molecular interactions critical for ligand optimization.
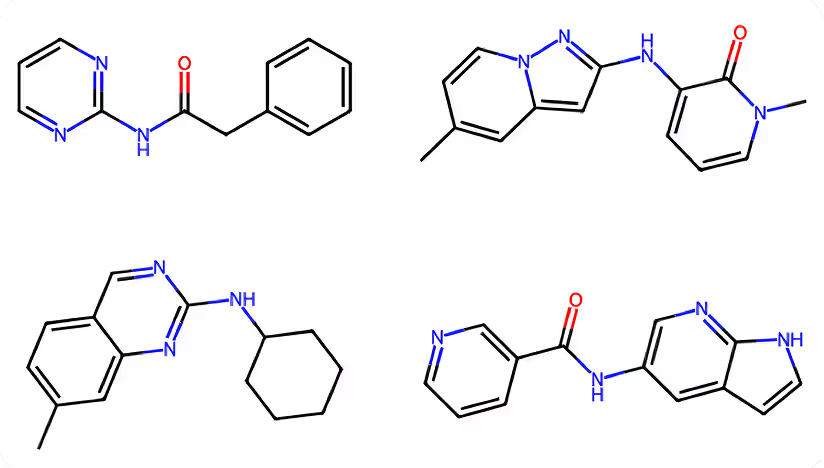
Scaffold Generation:- • The Scaffold Generation module was used to explore a diverse range of core structures.
- • A specific scaffold was selected for further development based on its favorable binding interactions and high synthetic accessibility.
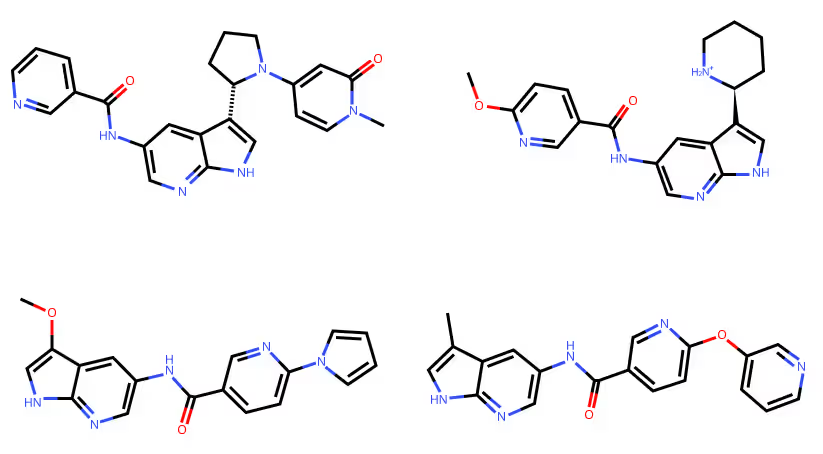
Molecular Expansion:- • We applied the Periphery Generation module to expand the selected scaffold and generate a diverse array of molecular candidates.
- • Medicinal chemistry insights guided the selection of the most promising compound, designated as INV-1, for synthesis and further evaluation.
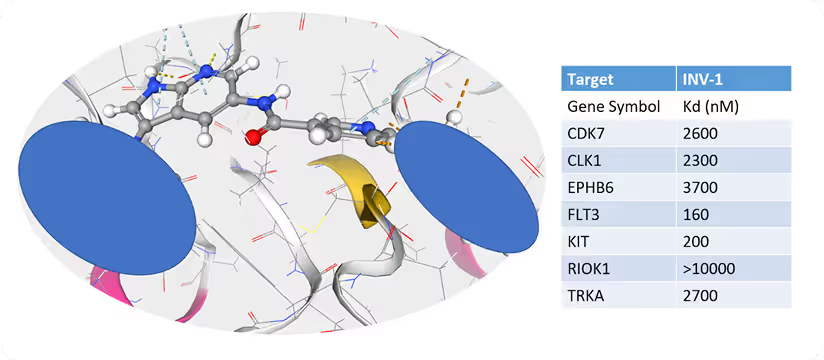
Experimental Validation:- • INV-1 was synthesized and tested in vitro for its inhibitory activity against FLT3 and other relevant kinases.
- • IC50 values were determined to assess the potency and selectivity of the compound.
Results
INV-1 demonstrated potent inhibition of FLT3 with an IC50 of 160 nM. The compound also exhibited activity against KIT (IC50 = 200 nM), another relevant kinase in AML, while maintaining weak inhibition against off-targets such as RIOK1 (>10,000 nM) and TRKA (2,700 nM). This favorable selectivity profile suggests that INV-1 holds significant promise as a lead compound for further therapeutic development.
Next steps
Lead Optimization:Further refinement of INV-1 to enhance potency, selectivity, and pharmacokinetic properties.
Biological Evaluation:Conduct additional in vitro and in vivo studies to evaluate efficacy, toxicity, and pharmacokinetics.
Model Enhancements:Incorporate advanced AI-driven validation techniques to improve predictive accuracy and streamline future discovery efforts.
This study underscores the potential of AI-assisted drug design to accelerate the identification and optimization of novel therapeutic candidates, providing a faster and more efficient pathway toward the development of targeted treatments for AML.